22+ Protons Neutrons Electrons Calculator
Solution 23 No the statement is not valid because atoms can be divided into subatomic particles called electrons protons and neutrons. 2020 at 322 pm.
Atomic Charge Calculator Calculator Academy
Web Stability of isotopes is affected by the ratio of protons to neutrons and also by presence of certain magic numbers of neutrons or protons which represent closed and filled quantum shells.

. Web In physical cosmology Big Bang nucleosynthesis abbreviated BBN also known as primordial nucleosynthesis is the production of nuclei other than those of the lightest isotope of hydrogen hydrogen-1 1 H having a single proton as a nucleus during the early phases of the UniversePrimordial nucleosynthesis is believed by most cosmologists to have. Web Theory predicts that about 1 neutron remained for every 6 protons with the ratio falling to 17 over time due to neutron decay. Web The Star Trek fictional universe contains a variety of weapons ranging from missiles the classic photon torpedo to melee primarily used by the Klingons a race of aliens in the Star Trek universe.
Web An X-ray or much less commonly X-radiation is a penetrating form of high-energy electromagnetic radiationMost X-rays have a wavelength ranging from 10 picometers to 10 nanometers corresponding to frequencies in the range 30 petahertz to 30 exahertz 3 10 16 Hz to 3 10 19 Hz and energies in the range 145 eV to 124 keVX-ray wavelengths. Must contain at least 4 different symbols. Thank u so much to.
Instead they are fixed within electronic orbitals. 602214076 1023 but the weight of each sample would be different. 1 molecule of methane contains 6 1 4 10 electrons 602 10 22 molecule will contain 10 602 10 22 602 10 23 electrons.
Its unit is a unified atomic mass and is denoted by the symbol u. This force is carried by gluons and binds quarks together to form larger particles. ASCII characters only characters found on a standard US keyboard.
Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. It acts upon the elements of the nucleus of the atom keeping neutrons and protons together. A nuclide of titanium Ti contains 22 protons and 26.
So a distinction is made between dose which is already in a location which is defined here as being background and the dose due to a. These quantum shells correspond to a set of energy levels within the shell model of the nucleus. Neils Bohr suspected that electrons revolved in quantized orbits.
Web There are 18 protons from the argon element. I has 53 protons 53 electrons and 78 neutrons. Web An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space.
Web Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off. Web Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleusAlpha particle emissions are generally produced in the process of alpha decay. Web Water is the chemical substance with chemical formula H 2 O.
As the lightest element of the boron group it has three valence electrons for forming covalent bonds resulting in many compounds such as boric acid the mineral sodium borate and. Web As discussed before Rutherford described an atom as consisting of a positive centre mass surrounded by orbiting electrons. Web Solution 22 The major drawback of Daltons atomic theory is that atoms were thought to be indivisible.
But it is not true since atoms are divisible. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5. Electrons however are not simply floating within the atom.
6 to 30 characters long. Write the complete electron configuration for each isotope. Atomic mass is the average mass of the protons neutrons and electrons in an atom.
Web That carbon atom is made up of 6 protons 6 neutrons and 6 electrons. In its amorphous form it is a brown powder. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Quarks form neutrons protons and other larger particles. Having suspected this Bohr worked on Rutherfords model and proved that particles couldnt occupy just any energy level.
Web Lithium is one example where the number of neutrons is not equal to the number of protons. Harshita September 26 2020 at 413 pm. If we set the proton mass to equal 1 unit then we get.
This gives us somewhere between 1022 and 10. Background radiation is defined by the International Atomic Energy Agency as Dose or dose rate or an observed measure related to the dose or dose rate attributable to all sources other than the ones specified. Web One mole of different substances contains the same number of particles ie.
Given the following identify the subatomic particles present. Microsoft describes the CMAs concerns as misplaced and says that. Heavier elements have more neutrons than protons in the nucleus.
Filled shells such as the filled shell of 50 protons for tin confers unusual. The protons and neutrons are held inside the nucleus while the electrons occupy up the rest of the atom. Nucleus of an Atom Examples.
Web The number of protons neutrons and electrons an atom has. The periodic table is required to solve these problems. A 024 g sample of a compound of oxygen and boron was found by analysis to contain 0096 g of boron and 0144 g of oxygen.
Alpha particles are a strongly ionizing form of radiation but when emitted by radioactive decay they have low penetration power and can be absorbed by a few. Co has 27 protons 27 electrons and 33 neutrons. There are a few more assumptions we have to make before we break out the calculator.
In its crystalline form it is a brittle dark lustrous metalloid. Web Since protons and neutrons account for almost all of the mass of the given atom. The negatively charged electrons which contribute little in terms of mass but are electrically equivalent to the protons in the nucleus orbit the positively charged core.
This is a free neutron denoted by the lower case n. This is believed to be correct because at a later stage the neutrons and some of the protons fused leaving hydrogen a hydrogen isotope called deuterium helium and other elements which can be measured. Web Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language.
Web The nucleus in the atoms Bohr model holds most of the atoms mass in its protons and neutrons. Web Boron is a chemical element with the symbol B and atomic number 5. Web How many protons neutrons and electrons are in atoms of these isotopes.
Web 01 mole has 602 10 22 molecules. A neutron is about 013 heavier than a proton and an electron is 1836 times lighter than a proton. As we know the number of protons neutrons and electrons in atoms of different elements differ consequently making the masses of those atoms different from each other.
The Star Trek franchise consists primarily of several multi-season television shows and a dozen movies as well as various video games and inspired merchandise. The proton neutron and electron can be represented by the following nuclide notations. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7.
There 18 electrons because it is neutral and 22 neutrons because 40 - 18 22. Web The strong force is the strongest of the four forces. 0 protons 1 neutrons 0 electrons.

Atomic Mass Calculator Calculate Neutrons Protons Electrons

Atomic Mass Calculator Calculate Neutrons Protons Electrons

U2hswwqeceak9m
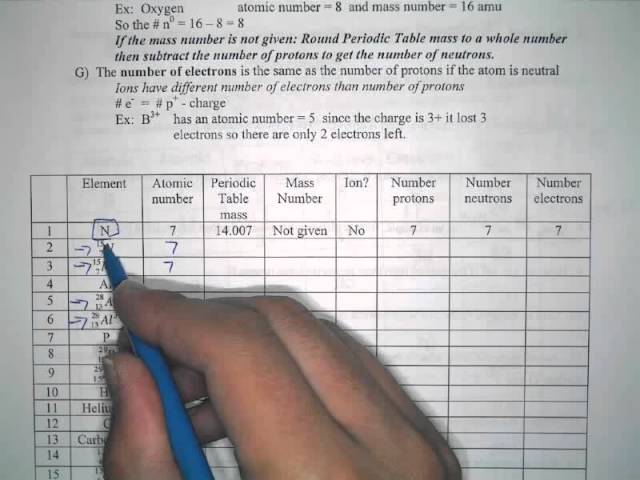
Proton Neutron Electron Calculation Practice Mov Youtube
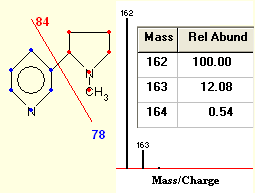
Isotope Distribution Calculator Mass Spec Plotter Isotope Abundance Graphs
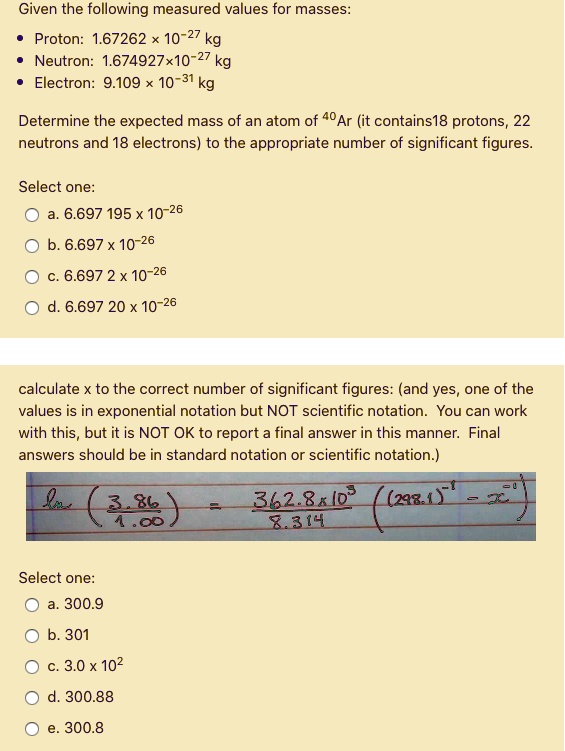
Solved Given The Following Measured Values For Masses Proton 1 67262 10 27 Neutron 1 674927 10 27 Kg Electron 9 109 10 31 Kg Determine The Expected Mass Of An Atom Of 40ar It Contains18 Protons 22 Neutrons

2 3 Calculating Atomic Masses Problems Chemistry Libretexts

How To Calculate Atomic Mass Practice Problems Youtube

How To Find The Number Of Protons Neutrons And Electrons

You Will Need Calculator Notebook Periodic Table Ppt Download
Yixdv1bddgiuxm

2 3 Calculating Atomic Masses Problems Chemistry Libretexts

Atom Calculator On The App Store

Worked Example Atomic Weight Calculation Video Khan Academy
2 3 Calculating Atomic Masses Chemistry Libretexts

Atom Calculator By Gorasiya Vishal Nanjibhai

Happy Friday Please Take Out Note Packet And Calculator Ppt Download